IT Mark Document Management adopt the ISO standard of documentation requirement for Record Management Procedure to our customer.
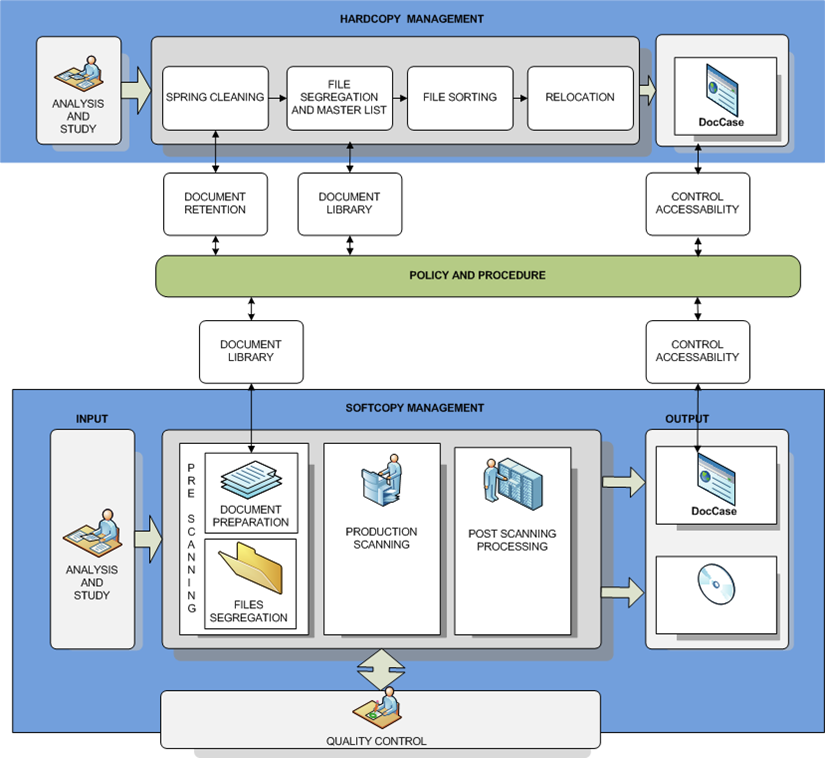 Document Management Procedure IT Mark
Document Management Procedure IT Mark
ISO 9001:2008 Documentation Requirements
ISO 9001:2008 clause 4.1 General requirements requires an organization to “establish, document, implement, and maintain a quality management system and continually improve its effectiveness in accordance with the requirements of this International Standard”
Clause 4.2.1 General explains that the quality management system documentation
All the documents that form part of the QMS have to be controlled in accordance with clause 4.2.3 of ISO 9001:2008, or, for the particular case of records, according to clause 4.2.4.
Clause 4.2 of ISO 9001:2008
The following comments are intended to assist users of ISO 9001:2008 in understanding the intent of the general documentation requirements of the International Standard.
a) Documented statements of a quality policy and objectives:
− Requirements for the quality policy are defined in clause 5.3 of ISO 9001:2008. The documented quality policy has to be controlled according to the requirements of clause 4.2.3. Note Organizations that are revising their quality policy for the first time, or in order to meet the amended requirements in ISO 9001:2008, should pay particular attention to clause 4.2.3 (c), (d) and (g).
− Requirements for quality objectives are defined in clause 5.4.1 of ISO 9001:2008. These documented quality objectives are also subject to the document control requirements of clause 4.2.3.
b) Documented procedures: − ISO 9001:2008 specifically requires the organization to have “documented procedures” for the following six activities:
i. 4.2.3 Control of documents
ii. 4.2.4 Control of records
iii. 8.2.2 Internal audit
iv. 8.3 Control of nonconforming product
v. 8.5.2 Corrective action
vi. 8.5.3 Preventive action